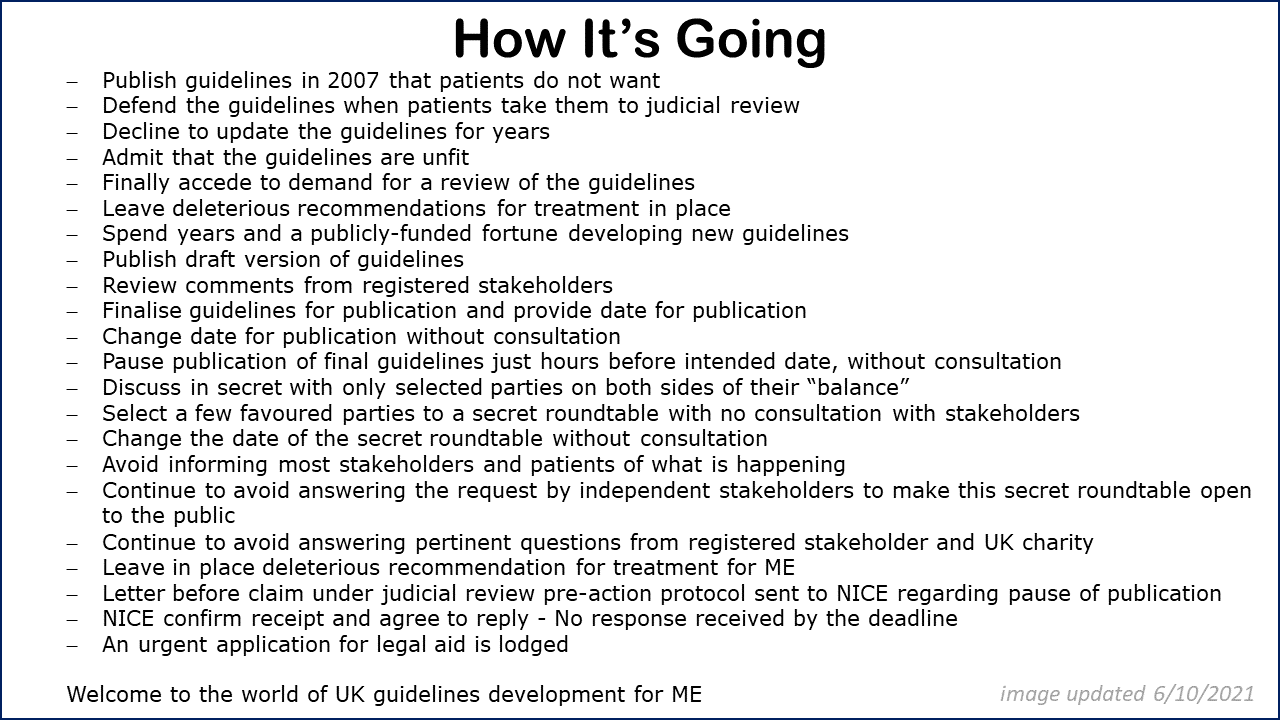
 On 29th September and following three weeks since we wrote our third letter to the CEO of NICE, Professor Gillian Leng, we
received a response to our concerns about the irregular pausing of the publication of the NICE guidelines for ME and the subsequent
clandestine meetings and lack of transparency from NICE when dealing with the future for people with ME and their families.
On 29th September and following three weeks since we wrote our third letter to the CEO of NICE, Professor Gillian Leng, we
received a response to our concerns about the irregular pausing of the publication of the NICE guidelines for ME and the subsequent
clandestine meetings and lack of transparency from NICE when dealing with the future for people with ME and their families.
In the interests of transparency we include that reply on this page - along with our response.
A reminder of the story so far
The reply from Professor Leng is here -
Thank you for your letter dated 08 September 2021, I am sorry for the delay in replying.
Firstly, I want to say that I am very aware that the pause in publication of the guideline has been difficult for people with ME and their families. However, we really want to ensure that the guideline makes a difference for patients, and unfortunately a pause to publication was necessary in the light of feedback from some key groups.
Communication
Regarding communication with stakeholders, we have made public statements and emailed registered stakeholders when there have been substantive updates to share.
Round table discussion
You ask a number of questions about the round table discussion. Our aim in holding this meeting is to further understand the issues raised before publication and to demonstrate the robust way the guideline has been developed. This will help us determine a way forward that will mean the guideline is supported to ensure effective implementation.
We know that there is significant interest in this meeting but to allow the independent chair to facilitate an effective discussion, there are limited places available for participants. This being the case, NICE decided to invite a range of stakeholders including national patient organisations and charities representing a variety of perspectives including those of adults, children, people with severe ME, an understanding of service delivery and an interest in research. We have also invited relevant professional societies, representatives from NHS England and NHS Improvement, NICE and the guideline committee. It is for those invited organisations to decide who they wish to represent them at the meeting.
We are sorry that we are not able to accommodate all those who wish to attend at this event.
In order to fully prepare for the meeting, we have held, or scheduled, an informal discussion with each organisation invited to the event.
It is important to note that the round table is for discussion of the issues raised that led to the pause in publication. It is not a decision-making forum. Any subsequent decisions will be made by NICE’s guidance executive.
We will publish the agenda and list of invited stakeholder groups in advance of the meeting.
NICE will not be recording the round table discussion. We want to encourage an open discussion and consider that recording the meeting might discourage some attendees from contributing freely.
We will make the minutes publicly available as soon as possible after the meeting.
In conclusion, I would add that changing clinical practice and clinical attitudes is one of the most challenging parts of NICE’s role. To make a real difference, guidelines need to be supported by all relevant stakeholders, so taking some extra time now to bring everyone together is an important step. I know the delay really matters, and I want to reach a resolution as quickly as possible. My hope is that by bringing key parties together, we can move forward in the spirit of collaboration and understanding.
Yours sincerely
Professor Gillian Leng CBE
Chief Executive
It takes time and effort to continue to try to interpret the actions of this publicly funded organisation - especially when, as with patients, we are kept in the dark regarding what is happening and when only a chosen few have been involved in the secret discussions away from the public gaze.
However, for the record, our reply carries our thoughts on the NICE response.
Rather than comment further we just place our reply in the public domain - below
Dear Professor Leng,
Thank you for taking the time to reply to our letters to you from 20 August, 3 September and 8 September.
You claim to be aware of the “difficulty” that the pause of the final NICE guidelines publication has caused to people with ME and their families.
One could be forgiven for thinking that NICE really does not understand what people with ME and their families have had to endure - since the 2007 NICE guidelines were forced onto patients and especially since the 17 August 2021 when you paused the publication of the agreed final guidelines just a few hours before the previously agreed date for publication.
Few, if any, patients seem to have identified any justifiable reason for there to be a pause.You state, “a pause to publication was necessary in the light of feedback from some key groups”.
Was this feedback not the standard process that was to be followed where all stakeholders could give comment on the draft guidelines and where NICE responded to all feedback?
This was part of the standard NICE guidelines development process.
Invest in ME Research provided feedback - as did those groups whom you now allow to dictate your actions.
The time for further discussion passed - as per NICE guidelines development regulations.Communication
We dispute your inference that NICE has effectively communicated what is happening with these guidelines to all stakeholders, let alone to patients.
Your statement that NICE “emailed registered stakeholders when there have been substantive updates to share” carries very little factual accuracy.
As a registered stakeholder Invest in ME Research has received just two communications from NICE (if one omits the automatic responses received from our other emails sent to you) – on 17 August when you announced the pause and on 27 August where you announced your round table to be arranged in September.
Indeed, your “substantive statements” have so far –
- failed to provide substantive detail to explain why it was necessary to depart from standard NICE guidelines development regulations by pausing the guidance publication with a sudden and very brief statement
- failed to provide any substantive explanation as to why secret meetings have been going on with some groups away from the gaze of other stakeholders, patients or the public
- avoided disclosing with whom you have been discussing these developments in secret
- failed to consult with all stakeholders in organising your round table with no substantive detail provided.
- failed to provide any substantive detail behind the reason to change the date for your round table without any warning
- avoided disclosing the agenda for your round table meeting
- avoided disclosing who would be invited to your round table meeting
- avoided disclosing why your favoured guests to this round table were invited and what selection criteria were used
- avoided disclosing whether the selection process (if there was one) for invitation to your round table followed standard NICE procedures
- avoided informing all stakeholders of what is happening
- avoided any opportunity for independent stakeholders to play any role in these further discussions
- avoided allowing patients to be appraised of what is happening
- avoided answering our question as to why you have chosen not to communicate what is happening to all stakeholders, via standard procedures, and at the same time?
- avoided providing any explanation as to how you can you be sure that all interests are being represented at your round table if all of the existing stakeholders have not been contacted for their views beforehand
- avoided answering why those who have been dismissed or resigned from the guidelines development group are able to continue to play any part in the processes concerning these guidelines
As a registered stakeholder, we have been given no opportunity to influence anything since early August and have felt left completely in the dark, gleaning information only from social media and from comments made by some of these groups whom you have invited to your round table.
Round table Discussions
Your behind the scenes discussions and selection of a minority of “interested parties” as "representatives" from the patient community perhaps achieved the objective of your stratagem of integrating an apparent veil of patient acceptance that could be pulled over any serious attempt for substantive discussions and explanation. This would then allow a clear path to accommodating those stakeholders who are resisting removal of GET as a recommendation from the guidelines.
The downside to that tactic is that it feeds speculation, continues to cast doubt on the trustworthiness of NICE (and some “interested parties”) and merely results in more confusion and anxiety from patients who know little of why this pause happened in the first place.
You write that your aim in holding your round table “is to further understand the issues raised before publication and to demonstrate the robust way the guideline has been developed”.
So what was the purpose of the official review phase once the draft guidelines were published?
Invest in ME Research may be more critical about NICE and the harm it has done than most establishment patient organisations but we have agreed to abide by the NICE development rules.
We have returned comments and we have hoped that NICE would listen and amend the guidelines where necessary. Similarly other stakeholders have returned their comments and the NICE development process responded to those commentsThat process was completed – for all.
There is no further requirement to demonstrate the “robust way the guideline has been developed”
NICE afforded no such pleasantry to people with ME when they produced guidelines that were almost universally criticised by the ME patient community in 2006-7.
All stakeholders in 2020 were aware of the guidelines development process when they reviewed the draft guidelines.The guidelines development took several years and an enormous amount of money (a sum of public funding that is routinely denied to biomedical research into ME).
A draft document was produced according to NICE's own guidelines development protocols and comments were invited from stakeholders, and then answered.
The final guidelines were given a publication date.
There is no reason to prolong this with further discussion.If your round table is not meant to be a 'decision-making' forum then why do you not just meet with those who have raised issues that led to the pause?
Pausing to hold a non-‘decision-making’ meeting is not only grossly unfair to all other stakeholders who have made no threats and responded in good faith. It is also unfair to patients and their families – that community to whom you profess to “really want to ensure that the guideline makes a difference”.
In any case, according to NICE’s own manual, issues raised “before publication” can no longer be discussed or changed.
Yet you breach your own regulations by doing just this.
Your round table lacks legitimacy no matter how many patient organisations you entice to be silent by extending invitations.The way forward is simply to publish the guidelines and then hold your meeting, if necessary, on how to implement them.
You write that - “We know that there is significant interest in this meeting but to allow the independent chair to facilitate an effective discussion, there are limited places available for participants.”
Firstly, there is not significant interest in your round table.
There is significant interest in publishing the final guidelines following your own due process and as NICE committed to do.
Do not confuse this. Other than those organisations looking for attention as “representatives” there is no clamour from patients to hold your round table meeting.If there was such a demand for your round table to be arranged then it is a very simple matter for patients and their families to be allowed to listen in to the meeting, as you did with your Public Board meeting of 15th September that was streamed online to the public, with the agenda already visible beforehand.
Your claim to have an independent chair for your round table may not have been questioned by your invitees to your round table.
Yet your surveillance (and that of your associates) of reactions on social media must have noted that your choice for chair for your round table has been questioned by many in the patient/carer community as lacking in partiality and having past associations to organisations and policies that have not been conducive to the welfare of people with ME.You write that “NICE decided to invite a range of stakeholders including national patient organisations and charities representing a variety of perspectives including those of adults, children, people with severe ME, …….and an interest in research".
Invest in ME Research is a UK charity and our supporters are patients, including “adults and children and people with severe ME”. As a charity funding and facilitating biomedical research into ME (and currently funding the only clinical trial for ME in the UK and one of only a handful in Europe.) then yes, we are also a charity that has “an interest in research”.
Yet you have ignored or been advised to ignore this charity and its supporters and inadvertently explain why when you later stated “To make a real difference, guidelines need to be supported by all relevant stakeholders” and refer to “key parties”.You do not view Invest in ME Research and its supporters as “relevant” or in any sense a key party to your tactics. That was an unnecessary disparagement to people suffering from the disease yet who have done so much to move things on despite the lack of establishment attention.
We repeat our assertion that there should be no different approach exhibited toward different “categories” of stakeholders.
How many other registered stakeholders have you ignored in a similar way just to arrange a meeting that enables you to paint the picture that you desire?Your comment that – “It is for those invited organisations to decide who they wish to represent them at the meeting.” implies that you will not guarantee that individuals who resigned or were "dismissed" from the guidelines working group would be allowed any role in future discussions of these guidelines, and certainly not in your planned round table.
What an indictment of this omnishambles!You may write and even believe that there is a wish to appear to “encourage an open discussion and consider that recording the meeting might discourage some attendees from contributing freely”
Yet the way that you have subverted your own guidelines development protocols means that you have removed the possibility for stakeholders and patients from contributing freely to anything more in this debacle.
Despite all of the above, Invest in ME Research has had no burning desire to attend your round table.
We care not for a perception of prestige by invitation.
What we do care about is transparency and fairness and democracy in dealing with patients' issues – all qualities that seem to have been in short supply since 17 August.In fact, we believe that your round table has no credible meaning, has been constructed with little adherence to any acceptable due process and therefore carries no legitimacy, and ought not to be afforded any legitimacy from the patient community.
If, as you say, it is not meant to be a decision-making forum then why is the meeting not open for all to listen – or just for those whose objections or threats you take more seriously than feedback from other stakeholders?
Any round table that you organise ought just to include those who object to publication, so that you can explain to them how robust your procedures have been – and that meeting ought to follow the publication of the guidelines, not precede it.
Summary
In summary, this is not the way that NICE should go about its business.
The guidelines process has descended into an unrepresentative, corrupted and irrelevant period of confusion, secrecy, miscommunication, intrigue - a damaging dog’s breakfast of mediocrity.
Secret discussions with preferred groups, breaches of your own guidelines development rules and regulations, perceived caving in to pressure from some establishment influences, selecting preferred groups to attend your round table and closing down discussions by asking compliant groups to sign an NDA for your round table, obscuring information from patients and their families, miscommunicating what is happening.
A veritable omnishambles.This is not what one should expect from a publicly funded organisation - especially one that exerts so much influence on patients' lives.
There is no reason to view your round table as carrying any legitimacy by going along with it and giving it support by default.
Patients do not have to support this forced imposition of compliance.
Nobody should legitimise further manipulation, fudging and double-dealing involving "interested parties" seemingly only representing themselves.
At NICE you need to do things differently and you need to do them better.
What you should do is –
- Publish the guidelines immediately, as previously agreed – warts and all
- Discuss with those who object to removal of GET as a recommendation at a time that follows the guidelines publication
We doubt you will listen.
While these games are played out with people's lives then deleterious recommendations from the current guidelines are still left in place allowing more harm to be done to people with ME.
Utterly appalling!We, for one, will not be a silent witness to this and we wish to put these views on record.
We will not happily sail down that river that you and your partners have tried to sell to patients.
While you leave your position at NICE at the end of this year then perhaps you may reflect that patients and their families are left to deal with the consequences of these manipulations and this intrigue – perhaps for years to come.
Yours Sincerely,
The chair and board of Invest in ME Research
Update 12/10/2021
Since our reply to the CEO of NICE on 30 September 2021 (above) two developments have occurred.
- A patient has sought and been granted legal aid to appeal to the NICE decision to pause the publication of the guideline
- NICE has finally (12 October) published the agenda for their illegitimate and secretive roundtable - two months after they took decision to pause the guidelines publication.
Although they have still not publicised (at the time of this update) the list of their chosen participants.
By far the most important development is the likelihood of NICE being taken to a judicial review – again - by a patient!
Lawyer Peter Todd, acting on behalf of his client (a person with ME), has been successful in seeking legal aid for a judicial review.
The purpose of the legal challenge was to prevent the unnecessary roundtable from taking place ahead of the publication of the guidelines.Having served notice to NICE he has then received a feeble response from NICE's expensive lawyers countering the claims.
IiMER claim no legal expertise and so any comment on the specific legal aspects associated with this reply are not possible.
We merely comment on the validity of what NICE and its willing accomplices are doing as it relates to the health of patients and their families and carers.
If NICE was in any way seeking to make things easier for patients then we would not be in this situation.
There is little point in examining the NICE lawyers' comments in any detail. Suffice to say that the response from the NICE lawyers indicates that NICE care less for the health of patients.The judicial process continues anyway.
In any case this is not new territory for people with ME. We have been here before with NICE.
As with the 2007 NICE guidelines, with the expensive lawyers that they employ to defend their decisions, they go against what we believe are the interests of patients.Let us consider that NICE has now developed two sets of guidelines for ME in fourteen years.
On both occasions NICE has been challenged via a judicial review – by patients!
What does this say about NICE and its fitness to perform this task - or the establishment organisations (including those claiming to support patients) that facilitate this dereliction of duty to make things better for patients?It should escape nobody's notice that a publicly-funded organisation is responsible for producing guidelines for ME, spends an exorbitant amount of public funds on the development of these guidelines, is then (for the second time in as many guidelines versions) criticised by ME patients who initiate a judicial review, and NICE then spends more public money financing its expensive lawyers to oppose patients.
This debacle would likely be seen as too deranged even to the worst B-movie script writers.
Surely only in dystopian UK could this be happening.Imagine how all of this money could have been used for funding research into the disease?!
As for the long awaited agenda for this secret roundtable meeting?
The agenda that has been published demonstrates the fallacy behind NICE’s commentary and reasoning regarding the pause.NICE's recent statement carried this from Professor Gillian Leng - CEO of NICE
“We are holding this roundtable to explain how the guideline was developed and the rationale behind the recommendations made and to hear and understand the concerns that have been raised. ”As we have mentioned before this has already been done – for all stakeholders – during the evaluation of the draft guidelines.
The agenda for the meeting is as follows:
- Introduction and rules of the meeting – Dame Carol Black
- Guideline production at NICE – Dr Paul Chrisp, director of the Centre for Guidelines at NICE
- Aim of the Myalgic encephalomyelitis/chronic fatigue syndrome: diagnosis and management guideline – Dr Peter Barry, chair of the ME/CFS guideline committee
- Discussion of issues raised: diagnosis, graded exercise therapy, children and young people, and cognitive behaviour therapy.
- Summary – Dame Carol Black.
Points 1 and 2 are irrelevant - there only to serve a structure for this illicit meeting..
Point 3 is obvious to all stakeholders – it was documented in the guidelines development process.
And so we get to the heart of all of this deception – point 4 – discussing further the removal of GET as a recommended treatment and the slight downplaying of the role of CBT.
This is why NICE has paused the guidelines and scheduled this meeting.Yet Leng has written to us that this secret roundtable for the chosen few is not a decision-making meeting – so what is the point of discussing further?
All stakeholders have had this opportunity during the draft guidelines review phase.
The guidelines development process was carried out and however many flaws there are still in the guidelines, as reviewed and commented on by Invest in ME Research and other stakeholders, that project achieved its goal according to the process that all stakeholders were aware of.
So we have a secret meeting organised, already described by the CEO of NICE as not being a "decision-making forum", convened for no purpose other than to placate one set of organisations who can see their golden eggs of GET and, to some extent, CBT being removed from the nest.
Unless, that is, there are plans to change the guidelines - as Professor Leng has stated "Any subsequent decisions will be made by NICE’s guidance executive.".
Any changes now would, of course, invalidate the whole development process and nullify the final guidelines.
It seems unlikely that the legal objections being constructed by lawyer Peter Dodds will prevent this secret roundtable from occurring, given the time remaining.
NICE has set this up with the inclusion of their favoured parties.
Those parties include some who issue statements that that they are ""not attending to discuss changes”.
So one might ask what are they attending for? To improve the guidelines!?
While claiming to want to have the guidelines published immediately they easily comply to NICE's wishes, hold secret behind the scenes meetings away from the view of patients and contribute to the lack of transparency in what is happening.
All this has served the attempts to legitimise this pause in publication, and the events following that pause, and merely adds to the impression that the only reason to wish to attend this roundtable is to bathe in the glow of self importance.
Graciously promising to update the ME community in the days following their roundtable means very little.
The deed is done already.What hypocrisy!
Such a high price to pay for an entry ticket to this meeting - and the price of that entry ticket may yet be passed on to patients in the long run.
But well played NICE!
Meanwhile, the old deleterious recommendations from the 2007 guidelines continue to circle like vultures over any newly diagnosed patients - waiting to inflict more harm.

